Micro- and nano-scale cell-implanted lasers
In the life sciences, the ability to track individual cells is critical to advance our understanding of many important biomedical questions; e.g. to study the function of neuronal networks, follow the inflammation response of immune cells, and unravel the way in which circulating tumour cells contribute to the formation of cancer metastasis.
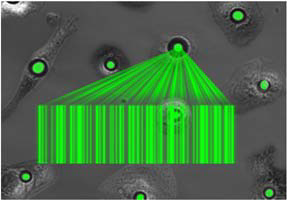
We have shown that living cell lasers can provide an unconventional solution to this problem. Rather than putting a cell into a laser resonator as was done previously [Nature Photonics, 2011], a microlaser can be placed inside a living cell [Nano Letters, 2015]. The extreme sensitivity of the lasing wavelength on the size of the laser and the local environment can be exploited to (1) bar-code and track individual cells (up to 1012 unique labels) [Scientific Reports, 2016], and (2) sense local environmental changes, e.g. to monitor the contraction of cardiac cell in vitro and in vivo [Nature Photonics, 2020].
We have extensive knowledge on micro and nano scale lasers, including on the optical gain and refractive index available from different classes of materials, and on the different resonator geometries available [see reviews: Nature Photonics, 2014; Chemical Reviews, 2017]. Based on this, organic whispering gallery mode lasers were identified as promising candidates for lasing within cells. We found that these can be small enough to enter cells through the endocytic pathway, and that they can be operated by optical pumping without causing noticeable cell damage. Very recently, we developed a second generation of intracellular lasers that are 500-fold smaller than the eukaryotic nucleus and have lasing thresholds 50-fold below the pulse energies typically used in in vivo two-photon microscopy [Nature Communications, 2018a]. We have also deployed intracellular lasers to sense the contraction of beating heart muscle cells, in vitro and in Zebra fish embryo, and have shown that laser-based sening can record signals with single-cell specificity deep in tissue, where most conventional microscopy methods struggle with severe light scattering [Nature Photonics, 2020].
Furthermore, we have developed a major variation of the bio laser concept – an ultra-thin membrane laser that can be transferred onto a variety of substrates, e.g. bank notes or contact lenses [Nature Communications, 2018b]. The pump threshold and emission intensity of this membrane laser are well within the eye safety limits for laser exposure. Therefore, a membrane laser on a contact lens can even be used in the human eye. The unique spectrum of the membrane lasers can be readily read out and thus, such an arrangement could serve as a wearable security tag.
